Landau Lab Summer Project
Virulent and Antimicrobial Amyloids in Infections and Neurodegeneration
Amyloids are protein fibers with unique and strong structures, known mainly in the context of neurodegenerative diseases. Surprisingly, amyloid fibers are secreted by species across kingdoms of life, including by microorganisms, and help their survival and activity. Our laboratory published the first molecular structures of functional bacterial amyloid fibrils, which serve as key “weapons” making infections more aggressive.
During the summer we invite you to explore one of the following projects:
1. Our findings exposed new routes for the development of novel antivirulence drugs, which we would like to peruse via structural and mechanistic understanding and screens of drug-like compounds.
2. We revealed that amyloids secreted by bacteria highly abundant in the microbiome and food sources show similarities in molecular structures to human amyloids involved in neurodegenerative diseases such as Alzheimer’s and Parkinson’s. We would like to explore the involvement of microbes in facilitating these diseases, similar to prion proteins transmitted by contaminated meat that elicit the Creutzfeldt-Jakob disease.
3. We identified peptides produced across species that provide antimicrobial protection that form amyloid fibrils and determined their first high-resolution structures. We would like to further explore this amyloid-antimicrobial link, which signifies a physiological role in neuroimmunity for human amyloids.
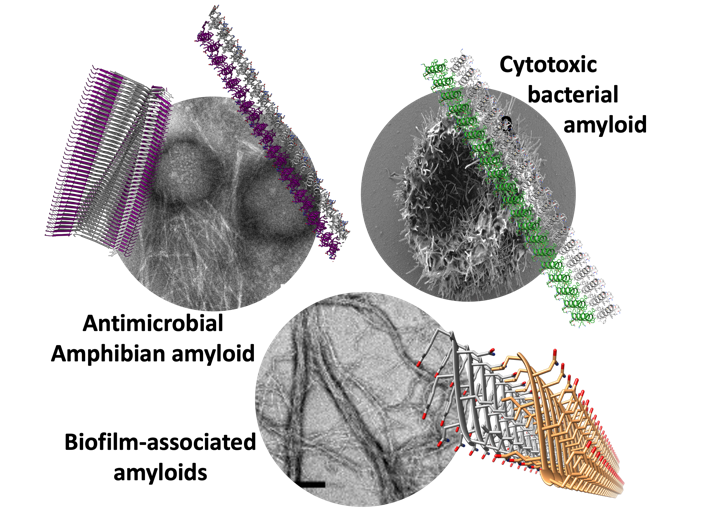
In the picture: Atomic structures of amyloid fibrils determined by X-ray crystallography and cryogenic electron microscopy (cryo-EM) of microbial and antimicrobial amyloids. A scanning electron micrograph shows cells damaged by a cytotoxic bacterial amyloid peptide, and transmission electron micrographs display fibrils of antimicrobial peptides covering bacterial cells, and of massive fibrils formed by biofilm-associated amyloids.
1. E. Tayeb-Fligelman, O. Tabachnikov, A. Moshe, O. Goldshmidt-Tran, M.R. Sawaya, N. Coquelle, J-P. Colletier, and M. Landau. Science 355(6327): 831-833; 2017
2. N. Salinas, A. Moshe, J-P. Colletier, and M. Landau. Nat. Commun. 9(1):3512; 2018.
3. S. Perov, O. Lidor, N. Salinas, N. Golan, E. Tayeb-Fligelman, M. Deshmukh, D. Willbold, and M. Landau. PLoS Pathog 15(8): e1007978; 2019
4. E. Tayeb-Fligelman, N. Salinas, O. Tabachnikov, and M. Landau. Structure S0969-2126(19)30445-9; 2020
5. Y. Engelberg and M. Landau. Nat. Commun. 11, 3894; 2020
6. N. Salinas, E. Tayeb-Fligelman, M. Sammito, D. Bloch, R. Jelinek, D. Noy, I. Uson, and M. Landau. PNAS 118 (3) e2014442118; 2021
7. Y. Engelberg, P. Ragonis-Bachar and M. Landau. Biomacromolecules 2022
8. R. Bücker, C. Seuring, C. Cazey, K. Veith, M. García-Alai, K. Grünewald, and M. Landau. The Cryo-EM Structures of two Amphibian Antimicrobial Cross-β Amyloid Fibrils. Nat. Commun 13: 4356; 2022
9. P. Ragonis-Bachar*, B. Rayan*, E. Barnea, Y.Engelberg, A. Upcher, M. Landau. Natural Antimicrobial Peptides Self-assemble as alpha/beta Chameleon Amyloids. Biomacromolecules, in press







