Associate Prof. Shenhav Cohen Lab Project For The Summer
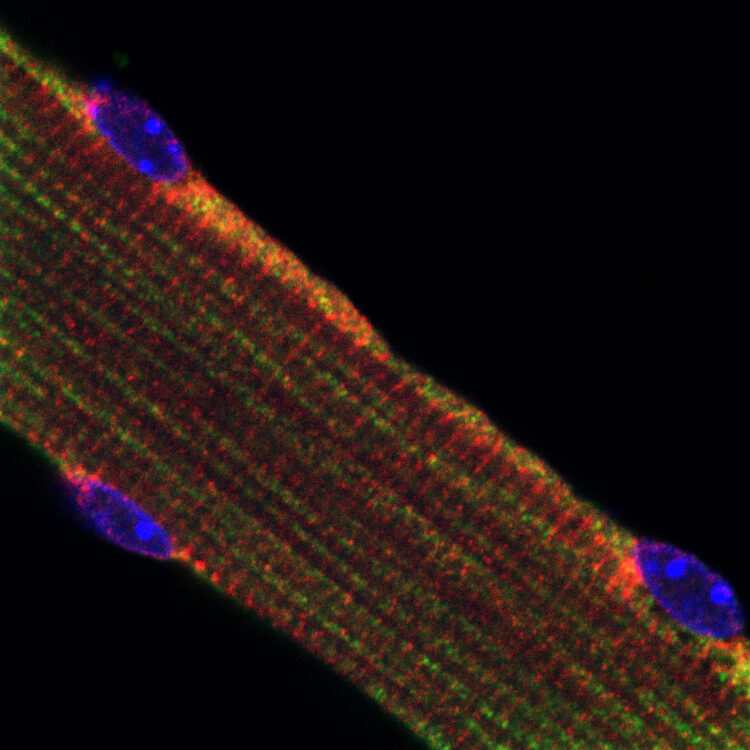
We study the molecular mechanisms that regulate muscle cell size. A reduction in muscle size during aging or disease results from excessive proteolysis by the proteasome and autophagy, leading to frailty, disability, and death. We strive to understand the catabolic pathways and cellular processes that precede and promote this debilitating loss of muscle mass in order to develop rational therapies to combat wasting. Our research encompasses different fields from physiology and metabolism to protein biochemistry and cell biology. Our goal is to advance a foundational understanding of diverse types of wasting, highlighting how cells retain and adapt their size to a changing physiological environment.
During the summer project, we invite you to choose one of the following areas :
1. Regulation of proteasome dynamics in atrophying muscles – We decipher the interplay between multiple transcription factors in inducing proteasome genes to adapt proteasome content to catabolic systemic states.
2. What protects the heart from fasting-induced atrophy? Skeletal muscles atrophy during systemic wasting. However, the heart is relatively protected, and atrophy to a much lesser extent than skeletal muscles. We investigate the underlying protective mechanisms, which appear to be independent of the ligand insulin.
3. Establishing an In Vitro Model for Disuse Atrophy– Disuse atrophy is the loss of muscle mass due to inactivity of innervated muscles (e.g. on exposure to microgravity in space, cast immobilization, bed-rest), or of denervated muscles (loss of nerve supply) as occurs naturally with aging, on spinal cord injuries, and in motor-neuron diseases (e.g. ALS, SMA). Animal models cannot be used for characterizing systematically the underlying cellular alterations that may be therapeutically targeted to prevent or slow disuse atrophy. Hence, we establish an in vitro model system for disuse using cells grown on Random Positioning Machine (RPM).







